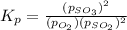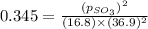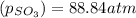Chemistry, 31.07.2019 23:10 yournerdybirdyp43oi3
At 900.0 k, the equilibrium constant (kp) for the following reaction is 0.345. 2so2 + o2 (g) →2 so3 (g) at equilibrium, the partial pressure of so2 is 36.9 atm and that of o2 is 16.8 atm. the partial pressure of so3 is atm.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:00
An example of technology is the a. addition of a side group to an organic molecule during synthesis. b. use of a new antibiotic to fight an infection. c. measurement of iron concentration in a water sample. d. study of atomic fusion reactions
Answers: 3


Chemistry, 22.06.2019 06:10
How many moles of gas are present if p=11 atm, v=12l, t=185k?
Answers: 1

Chemistry, 22.06.2019 14:30
An object resting on a table weighs 100 n. with what force is the object pushing on the table? with what force is the table pushing on the object? explain how you got your answer.
Answers: 3
You know the right answer?
At 900.0 k, the equilibrium constant (kp) for the following reaction is 0.345. 2so2 + o2 (g) →2 so3...
Questions

Mathematics, 22.12.2020 20:20

Biology, 22.12.2020 20:20


Mathematics, 22.12.2020 20:20


Geography, 22.12.2020 20:20


Chemistry, 22.12.2020 20:20

Advanced Placement (AP), 22.12.2020 20:20

Mathematics, 22.12.2020 20:20


English, 22.12.2020 20:20


Mathematics, 22.12.2020 20:20

Mathematics, 22.12.2020 20:20

Mathematics, 22.12.2020 20:20

Mathematics, 22.12.2020 20:20

Mathematics, 22.12.2020 20:20


English, 22.12.2020 20:20

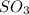 is, 88.84 atm
is, 88.84 atm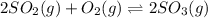
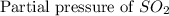 = 36.9 atm
= 36.9 atm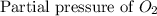 = 16.8 atm
= 16.8 atm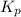 will be,
will be,