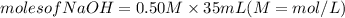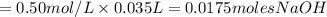Chemistry, 31.07.2019 22:10 richdakid26
If it requires 35.0 milliliters of 0.50 molar naoh to neutralize 25.0 milliliters of hcl, what is the concentration of the hcl solution? (3 points)
balanced equation: naoh + hcl yields nacl + h2o
select one:
a. 0.36 m hcl
b. 0.70 m hcl
c. 1.1 m hcl
d. 1.4 m hcl

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 15:10
Which statement describes the phase change that occurs when dry ice is placed in an open container at room temperature?
Answers: 1


Chemistry, 23.06.2019 03:00
Describe the properties of sodium, chlorine, and sodium chloride
Answers: 1

You know the right answer?
If it requires 35.0 milliliters of 0.50 molar naoh to neutralize 25.0 milliliters of hcl, what is th...
Questions



Mathematics, 28.03.2021 15:10

History, 28.03.2021 15:10




Mathematics, 28.03.2021 15:10


History, 28.03.2021 15:10


History, 28.03.2021 15:10



Chemistry, 28.03.2021 15:10

Mathematics, 28.03.2021 15:10

Mathematics, 28.03.2021 15:10

Mathematics, 28.03.2021 15:10


Chemistry, 28.03.2021 15:10

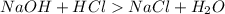
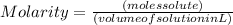 and its unit is mol/L
and its unit is mol/L