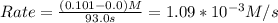Chemistry, 31.07.2019 21:30 navjitdosanjh20
For the gas phase decomposition of phosphine at 120 °c, the rate of the reaction is determined by measuring the appearance of h2. 4 ph3(g)p4(g) + 6 h2(g) at the beginning of the reaction, the concentration of h2 is 0 m. after 93.0 s the concentration has increased to 0.101 m. what is the rate of the reaction? (mol h2/l) /s

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:00
You encounter a solution that is acidic and you decide to test it by adding a small amount of a strong acid. the ph lowers slightly but is approximately unchanged, and still remains acidic. what can you say about the solution? a. it is a buffer solution. b. it is not a buffer solution it is a strong acid solution. d. the solution has been neutralized. e. the solution has excess acid present
Answers: 1

Chemistry, 22.06.2019 08:30
Which part of earth’s surface receives the most direct rays from the sun? a) equator b) ocean c) poles d) mountains
Answers: 2

Chemistry, 22.06.2019 11:00
Which statement is true about hcl? (5 points) select one: a. it is a salt because it increases the concentration of metallic ions. b. it is a salt because it is formed by the reaction of an acid and a base. c. it is an acid because it increases the concentration of hydroxyl ions. d. it is an acid because it increases the concentration of hydronium ions.
Answers: 1

Chemistry, 22.06.2019 20:00
I’m an electrically neutral atomic any element, there are equal numbers of
Answers: 2
You know the right answer?
For the gas phase decomposition of phosphine at 120 °c, the rate of the reaction is determined by me...
Questions

Biology, 02.08.2019 09:20

Chemistry, 02.08.2019 09:20

History, 02.08.2019 09:20




History, 02.08.2019 09:20

History, 02.08.2019 09:20

History, 02.08.2019 09:20

History, 02.08.2019 09:20


History, 02.08.2019 09:20

Biology, 02.08.2019 09:20


History, 02.08.2019 09:20

Biology, 02.08.2019 09:20

History, 02.08.2019 09:20

History, 02.08.2019 09:20


Business, 02.08.2019 09:20

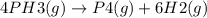
![Rate = +\frac{1}{6}*\frac{\Delta [H2]]}{\Delta t}](/tpl/images/0155/7784/3af01.png)
![Rate = +\frac{1}{6}*\frac{C2[H2]-C1[H2]}{\Delta t}](/tpl/images/0155/7784/69ced.png)