
The decomposition of hydrogen peroxide follows first order kinetics and has a rate constant of 2.54 x 10-4 s-1 at a certain temperature. if the concentration of hydrogen peroxide is 0.321 m after 855 s , what was the initial concentration of hydrogen peroxide at this temperature? 0.258 m0.399 m0.538 m0.677 m1.48 m

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:10
When 225mg of anthracene, c14h10(s), was burned in a bomb calorimeter the temperature rose by 1.75k. calculate the calorimeter constant. by how much will the temperature rise when 125mg of phenol, c6h5oh(s), is burned in the calorimeter under the same conditions? (δch< (c14h10,s)=–7061 kj mol−1.)
Answers: 3

Chemistry, 22.06.2019 07:30
Compare and contrast the bohr model and the electron cloud models of the atom.
Answers: 1

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 2

Chemistry, 22.06.2019 12:20
Achemistry student weighs out 0.306 g of citric acid (h3c6h5o7), a triprotic acid, into a 250 ml volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with 0.1000 m naoh solution. calculate the volume of naoh solution the student will need to add to reach the final equivalence point. be sure your answer has the correct number of significant digits.
Answers: 3
You know the right answer?
The decomposition of hydrogen peroxide follows first order kinetics and has a rate constant of 2.54...
Questions


Spanish, 17.03.2020 06:42

Spanish, 17.03.2020 06:42

History, 17.03.2020 06:42


Biology, 17.03.2020 06:43

Mathematics, 17.03.2020 06:43



Mathematics, 17.03.2020 06:43



Mathematics, 17.03.2020 06:43



Business, 17.03.2020 06:43


Mathematics, 17.03.2020 06:43


![k=\frac{2.303}{t}\log\frac{[A_o]}{[A]}](/tpl/images/0155/7548/f1041.png)
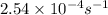
![[A_o]](/tpl/images/0155/7548/dc622.png) = initial amount of the reactant = ?
= initial amount of the reactant = ?![2.54\times 10^{-4}s^{-1}=\frac{2.303}{855s}\log \frac{[A_o]}{0.321}](/tpl/images/0155/7548/e899e.png)
![[A_o]=0.399M](/tpl/images/0155/7548/ee654.png)


