
Chemistry, 31.07.2019 20:30 alexandra2442
A12.22 gram sample of an organic compound containing only c, h, and o is analyzed by combustion analysis and 17.91 g co2 and 7.332 g h2o are produced. in a separate experiment, the molar mass is found to be 60.05 g/mol. determine the empirical formula and the molecular formula of the organic compound.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
What does x represent in the formula for the compound xcl4?
Answers: 1

Chemistry, 22.06.2019 09:40
How many grams of aluminum will there be in 98g of al2o3?
Answers: 1

Chemistry, 22.06.2019 14:30
Ahypothesis must be testable and falsifiable to be considered scientific a. trueb. false
Answers: 1

Chemistry, 22.06.2019 19:50
Which sentence from holes contains an implied personality trait? stanley and his parents had tried to pretend that he was just going away to camp for a while, just like rich kids do. he'd just been in the wrong place at the wrong time. stanley felt somewhat dazed as the guard unlocked his handcuffs and led him off the bus. stanley nodded to show he understood
Answers: 3
You know the right answer?
A12.22 gram sample of an organic compound containing only c, h, and o is analyzed by combustion anal...
Questions

Mathematics, 03.12.2019 20:31

Mathematics, 03.12.2019 20:31

Computers and Technology, 03.12.2019 20:31





Mathematics, 03.12.2019 20:31


English, 03.12.2019 20:31

Mathematics, 03.12.2019 20:31


Geography, 03.12.2019 20:31




Mathematics, 03.12.2019 20:31

Mathematics, 03.12.2019 20:31

Mathematics, 03.12.2019 20:31

Health, 03.12.2019 20:31

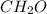 and
and  respectively.
respectively.
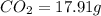

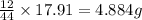 of carbon will be contained.
of carbon will be contained.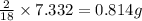 of hydrogen will be contained.
of hydrogen will be contained.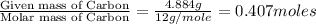


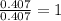
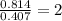


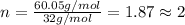

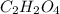 respectively.
respectively.

