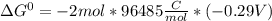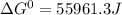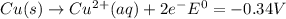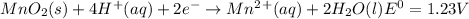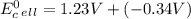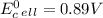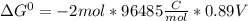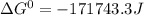Use tabulated electrode potentials to calculate ∆g° rxn for each reaction at 25 °c in kj.
(a)...


Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:50
Which of the following statements about acidic water is true? a. acid has no effect on the h,o molecules. b. the solution contains a larger number of oh ions than h,o ions. c. the solution contains a larger number of h,o ions than qh ions. d. the solution contains an equal number of h,o ions and oh ions. none of the above e.
Answers: 1

Chemistry, 22.06.2019 04:10
Answer from each drop-down menu. e characteristics of a borane molecule (bh). the lewis structure and table of electronegativities are given olecular shape is and the molecule is reset next erved. search e a
Answers: 2

Chemistry, 22.06.2019 04:30
How many moles of air are there in a human lung with a volume of 2.4 l at stp? explain your answer
Answers: 1

Chemistry, 22.06.2019 05:50
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3 -> a2cr(no3)3 + a3ag2so4
Answers: 1
You know the right answer?
Questions

Chemistry, 16.11.2019 04:31

English, 16.11.2019 04:31


Mathematics, 16.11.2019 04:31

Mathematics, 16.11.2019 04:31

History, 16.11.2019 04:31

English, 16.11.2019 04:31



Mathematics, 16.11.2019 04:31


English, 16.11.2019 04:31





Business, 16.11.2019 04:31

English, 16.11.2019 04:31

Mathematics, 16.11.2019 04:31

Mathematics, 16.11.2019 04:31

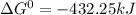 , (b)
, (b) 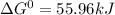 and (c)
and (c) 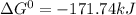
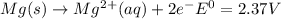
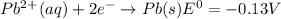
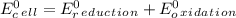
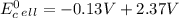
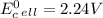
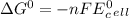
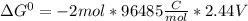
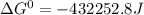
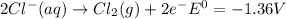
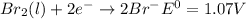
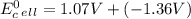
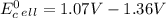
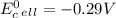
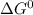 same as we did for part a.
same as we did for part a.