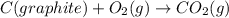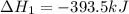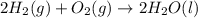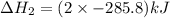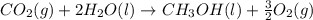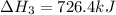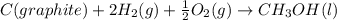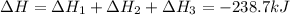Calculate δh for the reaction: c(graphite) + 2h 2(g) + 1/2 o 2(g) => ch 3oh(l) using the following information: c(graphite) + o 2 => co 2(g) δh o = -393.5 kj h 2(g) + 1/2 o 2 => h 2o(l) δh o = -285.8 kj ch 3oh (l) + 3/2 o 2(g) => co 2(g) + 2h 2o(l) δh o = -726.4 kj a. +238.7 kj b. -238.7 kj c. +548.3 kj d. -548.3 kj e. +904.5 kj

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:30
Consider the four elements above. which one of these elements will combine with oxygen in a 1: 1 ratio?
Answers: 3

Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical solutions?
Answers: 1

Chemistry, 22.06.2019 17:40
How much heat is added if 0.814g of water increase in temperature by 0.351 degree c?
Answers: 3

Chemistry, 22.06.2019 22:30
3.09 lab: reaction of metals 1 which combinations of substances resulted in a chemical change? for each metal that participated in a chemical change, write the type of metal it is, based on your examination of the periodic table. were there any metallic compounds that did not react with either the acid or the base? write the type of metal, based on your examination of the periodic table. make a general statement about the reactivity of the metals in this experiment.
Answers: 1
You know the right answer?
Calculate δh for the reaction: c(graphite) + 2h 2(g) + 1/2 o 2(g) => ch 3oh(l) using the follow...
Questions

Mathematics, 19.12.2020 01:00








Mathematics, 19.12.2020 01:00

Arts, 19.12.2020 01:00


Mathematics, 19.12.2020 01:00

Physics, 19.12.2020 01:00

Business, 19.12.2020 01:00

History, 19.12.2020 01:00


Mathematics, 19.12.2020 01:00



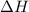 for the given reaction is -238.7 kJ
for the given reaction is -238.7 kJ