
Chemistry, 31.07.2019 19:10 brianlykid3042
Calculate the percent ionization of formic acid (hco2h) in a solution that is 0.125 m in formic acid. the ka of formic acid is 1.77 ⋅ 10-4. calculate the percent ionization of formic acid (hco2h) in a solution that is 0.125 m in formic acid. the ka of formic acid is 1.77 10-4. 0.859 0.0180 3.79 2.25 ⋅ 10-5 6.94

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Y=‐1x + 7 if y has a value of ‐24 what is the value of x?
Answers: 1

Chemistry, 22.06.2019 09:30
The chart shows the bid provided by four contractors to complete a job. which contractor is the most cost-effective?
Answers: 3

Chemistry, 22.06.2019 18:20
Which reason best explains why metals are malleable? a)because they have delocalized electrons b)because they have localized electrons c)because they have ionic bonds d)because they have rigid bonds
Answers: 2

Chemistry, 22.06.2019 21:30
Liquid ammonia is produced at high temperatures and under great pressure in a tank by passing a mixture of nitrogen gas and hydrogen gas over an iron catalyst. the reaction is represented by the following equation. n2(g) + 3h2(g) → 2nh3(g) changing all but one experimental condition will affect the amount of ammonia produced. that condition is a) increasing the concentration of both reactants b) changing the temperature within the tank c) decreasing the pressure within the tank. d) increasing only the amount of nitrogen present.
Answers: 1
You know the right answer?
Calculate the percent ionization of formic acid (hco2h) in a solution that is 0.125 m in formic acid...
Questions

Mathematics, 05.04.2020 00:38

English, 05.04.2020 00:38

Mathematics, 05.04.2020 00:38

Mathematics, 05.04.2020 00:38


Mathematics, 05.04.2020 00:38


Spanish, 05.04.2020 00:38

History, 05.04.2020 00:38

Mathematics, 05.04.2020 00:39

Chemistry, 05.04.2020 00:39

English, 05.04.2020 00:39


Mathematics, 05.04.2020 00:39



Mathematics, 05.04.2020 00:39


English, 05.04.2020 00:40

Mathematics, 05.04.2020 00:40

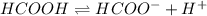
![\frac{[HCOO^{-}][H^{+}]}{[HCOOH]}=K_{a}(HCOOCH)](/tpl/images/0155/4252/45db7.png)
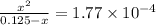
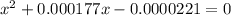
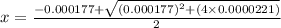
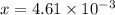 M
M![[H^{+}]=4.61\times 10^{-3}M](/tpl/images/0155/4252/38c16.png)
![\frac{[H^{+}]}{initial concentration of HCOOH}\times 100](/tpl/images/0155/4252/17262.png) =
= 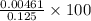 = 3.69%
= 3.69%

