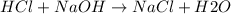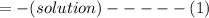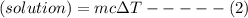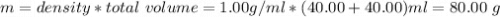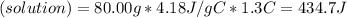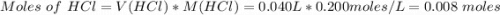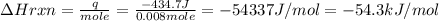When 40.0 ml of 0.200 m hcl at 21.5°c is added to 40.0 ml of 0.200 m naoh also at 21.5°c in a coffee-cup calorimeter, the temperature of the resulting solution rises to 22.8°c. assume that the volumes are additive, the specific heat of the solution is 4.18 jg -1°c -1 and that the density of the solution is 1.00 g ml -1 calculate the enthalpy change, δh in kj for the reaction:

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 10:00
Main expenses you plan on making payments on a new car too. you want to spend 15% of your monthly net pay on the car payment, insurance, registration, and taxes combined. what is your monthly car allowance? $149.46 $298.91 $448.37 $597.83
Answers: 3

Chemistry, 22.06.2019 14:40
Pastoral farming is best described as a. a method of raising livestock and moving herds b. an african method of agriculture c. a method of cultivating crops on poor soils d. a common method of desert farming select the best answer from the choices provided a b c d
Answers: 2

Chemistry, 22.06.2019 15:30
The identities of substances are the same before and after which type of change
Answers: 1
You know the right answer?
When 40.0 ml of 0.200 m hcl at 21.5°c is added to 40.0 ml of 0.200 m naoh also at 21.5°c in a coffee...
Questions

Arts, 10.05.2021 16:30




Mathematics, 10.05.2021 16:30


History, 10.05.2021 16:30


Mathematics, 10.05.2021 16:30

Arts, 10.05.2021 16:30



Physics, 10.05.2021 16:30


English, 10.05.2021 16:30

Mathematics, 10.05.2021 16:30



Computers and Technology, 10.05.2021 16:30

Mathematics, 10.05.2021 16:30