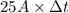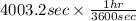Chemistry, 31.07.2019 18:20 chanel2371
Chromium plating can be applied by electrolysis to objects according to the following unbalanced half-reaction: cr2o72- + e- + h+→ cr(s) + h2ohow long (in hours) would it take to apply a chromium plating 0.010 mm thick to a car bumper with a surface area of 0.25 m2 in a cell with a current of 25.0 a? the density of chromium is 7.19 g/cm3.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Which actions would increase the rate at salt dissolves in water? stir the water? crush the salt? use less water? heat the water? cool the salt
Answers: 3

Chemistry, 22.06.2019 09:00
Which two scientific disciplines are bridged by pharmaceutical drugs? chemistry and forensics chemistry and medicine biology and forensics biology and criminology
Answers: 3

Chemistry, 22.06.2019 14:00
Which of the following is true about a carbonated soft drink? . the carbon dioxide is the solvent, and water is the solute.. the water is the solution, and carbon dioxide is the solvent.. the carbon dioxide is the solution, and the water is the solvent.. the water is the solvent, and the carbon dioxide is the solute.. .
Answers: 1

Chemistry, 22.06.2019 16:00
Answer asap : ( a. how does mucus prevent the entry of pathogens? b. describe two ways white blood cells protect us from pathogens.
Answers: 1
You know the right answer?
Chromium plating can be applied by electrolysis to objects according to the following unbalanced hal...
Questions

Biology, 28.10.2020 17:50

Biology, 28.10.2020 17:50

Mathematics, 28.10.2020 17:50







Computers and Technology, 28.10.2020 17:50


Biology, 28.10.2020 17:50

History, 28.10.2020 17:50

Social Studies, 28.10.2020 17:50



Mathematics, 28.10.2020 17:50

History, 28.10.2020 17:50

English, 28.10.2020 17:50

Mathematics, 28.10.2020 17:50

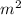 , current (I) = 25 A
, current (I) = 25 A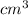
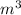 =
= 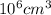
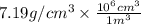
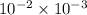 =
= 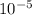 m.
m.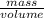
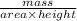
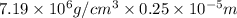
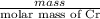
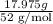
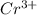 is 579000 C
is 579000 C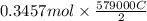 = 100080 C.
= 100080 C. 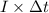
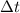 = time in seconds
= time in seconds