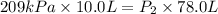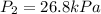Chemistry, 31.07.2019 17:20 houtchhaytang
Acylinder is filled with 10.0l of gas and a piston is put into it. the initial pressure of the gas is measured to be 209.kpa. the piston is now pulled up, expanding the gas, until the gas has a final volume of 78.0l. calculate the final pressure of the gas. be sure your answer has the correct number of significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. the values of phosphorous acid are 1.30 6.70 calculate the ph for each of the given points in the titration of 50.0 ml of 1.5 m h3po3(aq) with 1.5 m koh(aq) .
Answers: 3

Chemistry, 22.06.2019 10:40
Asolid that forms and separates from a liquid mixture is called
Answers: 2

Chemistry, 22.06.2019 11:20
Sodium nitrite (nano2) reacted with 2−iodooctane to give a mixture of two constitutionally isomeric compounds of molecular formula c8h17no2 in a combined yield of 88%. draw reasonable structures for these two isomers. click the "draw structure" button to launch the drawing utility. place the two compounds in the appropriate boxes below.
Answers: 1

Chemistry, 22.06.2019 17:30
Take a look at this dandelion. the yellow flower on the right is pollinated and the seeds on the left are transported by
Answers: 2
You know the right answer?
Acylinder is filled with 10.0l of gas and a piston is put into it. the initial pressure of the gas i...
Questions

Biology, 09.09.2021 22:00

Arts, 09.09.2021 22:00


History, 09.09.2021 22:00

World Languages, 09.09.2021 22:00

Mathematics, 09.09.2021 22:00



Mathematics, 09.09.2021 22:00



History, 09.09.2021 22:00


Mathematics, 09.09.2021 22:00


Social Studies, 09.09.2021 22:00



Mathematics, 09.09.2021 22:00

Mathematics, 09.09.2021 22:00

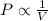
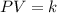
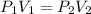
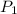 = initial pressure of the gas = 209 kPa
= initial pressure of the gas = 209 kPa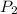 = final pressure of the gas = ?
= final pressure of the gas = ?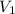 = initial volume of the gas = 10.0 L
= initial volume of the gas = 10.0 L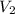 = final volume of the gas = 78.0 L
= final volume of the gas = 78.0 L