
Chemistry, 31.07.2019 04:30 marieknight689
Consider the balanced chemical equation that follows. you are asked to determine how many moles of water you can form from 4 moles of hydrogen and excess oxygen. 2h2(g)+o2(g)→2h2o(l) which of the following shows calculations for a correct way to solve this problem? view available hint(s) consider the balanced chemical equation that follows. you are asked to determine how many moles of water you can form from 4 moles of hydrogen and excess oxygen. which of the following shows calculations for a correct way to solve this problem? 4 mol h2×2 mol h2o2 mol h2=4 mol h2o 4 mol h2×2 mol h2o1 mol o2=8 mol h2o 2 mol h2×2 mol h22 mol h2o=2 mol h2o

Answers: 3


Another question on Chemistry


Chemistry, 21.06.2019 20:00
Smog is the term used to describe the combination of fog and smoke
Answers: 1

Chemistry, 22.06.2019 10:30
Skills of homo sapiens were found an excavation. the skulls were preserved because the bodies were frozen. so, these fossils are (blank) fossils.the image shows the evolution of skulls beginning 2 to 3 million years ago. based on the image, modern human skulls(blank) ape skulls.
Answers: 1

Chemistry, 22.06.2019 18:30
Which of the following nuclei would be the least stable a 2 protons, 2 neutrons b 1 proton 1 neutron c 1 proton 3 neutrons d 1 proton 2 neutrons
Answers: 3
You know the right answer?
Consider the balanced chemical equation that follows. you are asked to determine how many moles of w...
Questions


Mathematics, 24.02.2021 01:30


English, 24.02.2021 01:30

Mathematics, 24.02.2021 01:30


Mathematics, 24.02.2021 01:30

History, 24.02.2021 01:30



Geography, 24.02.2021 01:30


Mathematics, 24.02.2021 01:30



Mathematics, 24.02.2021 01:30




Mathematics, 24.02.2021 01:30

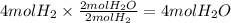
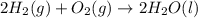
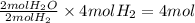 of water.
of water.

