Chemistry, 30.07.2019 22:20 palcochran1313
Given the following balanced reaction between liquid ammonia and oxygen gas to produce nitrous oxide gas and water, how many grams of water, h2o, are produced from 317 grams of ammonia and excess oxygen? (to find the molar mass in the problem use the periodic table and round the mass to the hundreds place for calculation).
(a) 224 g
(b) 335 g
(c) 503 g

Answers: 3


Another question on Chemistry


Chemistry, 23.06.2019 05:40
Why is it incorrect to balance a chemical equation by changing the subscripts? explain.
Answers: 2

Chemistry, 23.06.2019 10:30
Using the periodic table, complete the following. element: hydrogen symbol: h₂ molecular weight: g mass of one mole: g/mol
Answers: 2

Chemistry, 23.06.2019 10:50
Gene expression control that occurs during the generation of rna is a. controlled at transcription b. control before transcription c. controlled after transcription d. controlled after translation
Answers: 3
You know the right answer?
Given the following balanced reaction between liquid ammonia and oxygen gas to produce nitrous oxide...
Questions

Advanced Placement (AP), 19.10.2020 06:01



English, 19.10.2020 06:01

History, 19.10.2020 06:01

Mathematics, 19.10.2020 06:01

Biology, 19.10.2020 06:01


English, 19.10.2020 06:01





Mathematics, 19.10.2020 06:01

Mathematics, 19.10.2020 06:01


Mathematics, 19.10.2020 06:01

Mathematics, 19.10.2020 06:01

Mathematics, 19.10.2020 06:01

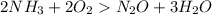
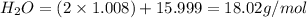
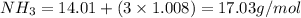
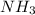 to moles
to moles 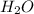 by using mole ratio of
by using mole ratio of 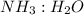 i.e., 2 : 3
i.e., 2 : 3
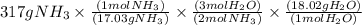
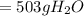 is formed.
is formed.
 is
is