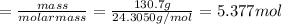Chemistry, 30.07.2019 04:10 luvpeaceandsocc6312
Assuming an efficiency of 49.50%, calculate the actual yield of magnesium nitrate formed from 130.7 g of magnesium and excess copper(ii) nitrate. mg+cu(no3)2⟶mg(no3)2+cu actual yield:

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:00
When you mate two plants together the terms is called? answer it fast as possible plz! i have a test tomorrow!
Answers: 1

Chemistry, 22.06.2019 08:00
What is the molarity of 60.0 grams of naoh dissolved in 750 milliliters of water? a) 1.1 m b) 2.0 m c) 12 m d) 75 m
Answers: 1

Chemistry, 22.06.2019 09:40
Apiece of copper has a temperature of 75.6 0c. when the metal is placed in 100.0 grams of water at 19.1 0c, the temperature rises by 5.5 0c. what is the mass of the metal?
Answers: 1

Chemistry, 22.06.2019 19:30
Which liquid (h2o, h2o + soap, or h2o + salt) has the strongest cohesion and adhesion? (need now plz)
Answers: 1
You know the right answer?
Assuming an efficiency of 49.50%, calculate the actual yield of magnesium nitrate formed from 130.7...
Questions




Computers and Technology, 16.11.2020 22:20

Mathematics, 16.11.2020 22:20


Social Studies, 16.11.2020 22:20


Mathematics, 16.11.2020 22:20

Arts, 16.11.2020 22:20

Mathematics, 16.11.2020 22:20


Mathematics, 16.11.2020 22:20



Biology, 16.11.2020 22:20

Mathematics, 16.11.2020 22:20

History, 16.11.2020 22:20

Advanced Placement (AP), 16.11.2020 22:20

Law, 16.11.2020 22:20