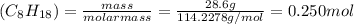Chemistry, 29.07.2019 23:30 Gladistshiala267
Consider the combustion reaction for octane (c8h18), which is a primary component of gasoline. 2c8h18+25o2⟶16co2+18h2o how many moles of co2 are emitted into the atmosphere when 28.6 g c8h18 is burned?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Choose all the answers that apply. as ocean depth increases, temperature decreases temperature increases pressure increases pressure decreases salinity increases density increases
Answers: 2

Chemistry, 22.06.2019 12:30
Acontrol during an experiment. might change remains constant does not exist does change
Answers: 1

Chemistry, 22.06.2019 13:00
How many moles of sulfur dioxide are produced when 4.38 moles of oxygen completely react with iron (iv) sulfide
Answers: 2

Chemistry, 22.06.2019 18:30
Which rate indicates the number of children that would be born per woman if she were to live to the end of her child bearing years
Answers: 2
You know the right answer?
Consider the combustion reaction for octane (c8h18), which is a primary component of gasoline. 2c8h1...
Questions

Mathematics, 05.12.2019 08:31


Mathematics, 05.12.2019 08:31

Mathematics, 05.12.2019 08:31



Chemistry, 05.12.2019 08:31

Mathematics, 05.12.2019 08:31




Mathematics, 05.12.2019 08:31

Chemistry, 05.12.2019 08:31



Chemistry, 05.12.2019 08:31

Mathematics, 05.12.2019 08:31

Mathematics, 05.12.2019 08:31

History, 05.12.2019 08:31