
Chemistry, 29.07.2019 23:20 adityamahesh2002
Calculate the total mass of the protons and electrons in 19 9f. use 1.007825 amu as the mass of 11h (mass of a proton and an electron). express your answer in atomic mass units to 6 significant figures.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:30
Which statement is true about this reaction? 14n+1h 15o it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2

Chemistry, 22.06.2019 17:40
Areaction in which products can react to re-form reactants is
Answers: 1

Chemistry, 22.06.2019 19:30
Use the periodic table to find the molar mass of each element. molar mass h = g/mol molar mass s = g/mol molar mass o = g/mol
Answers: 3

Chemistry, 22.06.2019 20:10
Suppose you mix one mole of sulfuric acid (h2so4) with 1 mole of sodium hydroxide(naoh). why does the ph of the solution remain below 7? ( explain so i can get better understanding! )
Answers: 2
You know the right answer?
Calculate the total mass of the protons and electrons in 19 9f. use 1.007825 amu as the mass of 11h...
Questions



English, 10.05.2021 17:30











Biology, 10.05.2021 17:30


Health, 10.05.2021 17:30

Mathematics, 10.05.2021 17:30



Mathematics, 10.05.2021 17:30

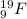 atom there are total 9 protons and number of neutrons is 19 - 9 = 10.
atom there are total 9 protons and number of neutrons is 19 - 9 = 10.

