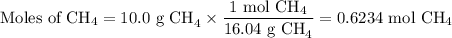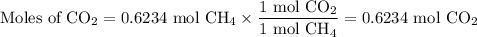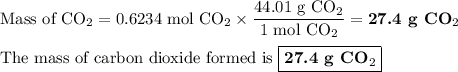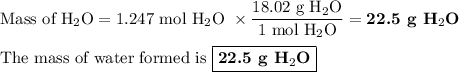Chemistry, 29.07.2019 22:20 milkshakegrande101
When 10.0 grams of ch4 reacts completely with 40.0 grams of o2 such that there are no reactants left over, 27.5 grams of carbon dioxide are formed. how many grams of water are formed? ch4+ 2o2 → co2 + 2h2o

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:20
Identify and describe the three ways that mutations affect organisms.
Answers: 1

Chemistry, 22.06.2019 06:00
The tilt of the earth's axis of rotation is responsible for the a) ocean's tides. b) size of the moon. c) brightness of stars. d) earth’s seasons.
Answers: 1

Chemistry, 22.06.2019 22:10
Which aqueous solution of ki freezes at the lowest temperature? 1) 1 mol of ki in 500. g of water 2) 2 mol of ki in 500. g of water 3) 1 mol of ki in 1000. g of water 4) 2 mol of ki in 1000. g of water
Answers: 3

Chemistry, 23.06.2019 01:00
Heat energy, carbon dioxide, and water are released through which process? a. photosynthesis b. depolymerization c. digestion d. cellular respiration
Answers: 1
You know the right answer?
When 10.0 grams of ch4 reacts completely with 40.0 grams of o2 such that there are no reactants left...
Questions

Advanced Placement (AP), 09.07.2019 22:00


Computers and Technology, 09.07.2019 22:00

English, 09.07.2019 22:00


Computers and Technology, 09.07.2019 22:00

Biology, 09.07.2019 22:00

Mathematics, 09.07.2019 22:00

Health, 09.07.2019 22:00

History, 09.07.2019 22:00


Chemistry, 09.07.2019 22:00



Mathematics, 09.07.2019 22:00


Biology, 09.07.2019 22:00