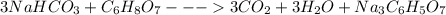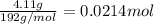Antacids, such as alka-seltzer, use the reaction of sodium bicarbonate with citric acid in water solution to produce a fizz as follows: 3nahco3 + c6h8o7 → 3co2 + 3h2o + na3c6h5o7 if 4.11 g of the citric acid (c6h8o7, mw = 192 g/mol) react with excess sodium bicarbonate (nahco3), how many grams of carbon dioxide (co2, mw = 44 g/mol) are formed as the solution fizzes?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:00
Which of the following is not a good technique for managing used oil? a) have specific, labeled catch pans available for technicians who are collecting oil b) spills in your shop and any releases on pavement or outside should be poured down a drain c) do not use oil containers for antifreeze or other non-similar fluids d) be prepared for oil spills with the proper absorbents
Answers: 1

Chemistry, 22.06.2019 05:30
Modern weaponry has increased the number of deaths in wars and violent conflicts.
Answers: 3

Chemistry, 22.06.2019 05:50
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3 -> a2cr(no3)3 + a3ag2so4
Answers: 1

Chemistry, 22.06.2019 07:00
The variability in marine salinity between habitats does not impact the fish living there. select the best answer from the choices provided t f
Answers: 1
You know the right answer?
Antacids, such as alka-seltzer, use the reaction of sodium bicarbonate with citric acid in water sol...
Questions

Mathematics, 25.10.2021 18:50


Biology, 25.10.2021 18:50


Mathematics, 25.10.2021 18:50


English, 25.10.2021 18:50

English, 25.10.2021 18:50

History, 25.10.2021 18:50

Mathematics, 25.10.2021 18:50

Mathematics, 25.10.2021 18:50

Computers and Technology, 25.10.2021 18:50

English, 25.10.2021 18:50

Social Studies, 25.10.2021 18:50

Mathematics, 25.10.2021 18:50

History, 25.10.2021 18:50

English, 25.10.2021 18:50

Spanish, 25.10.2021 19:00

Mathematics, 25.10.2021 19:00

English, 25.10.2021 19:00