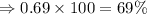Chemistry, 29.07.2019 21:10 Mexicochan
Copper exists naturally as two isotopes copper-63 with a mass of 62.93 amu and copper-65 with a mass of 64.93 amu. the average mass of copper is 63.55 amu. what is the approximate abundance of copper-63 in nature?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:00
2h2s + 3o2 2so2 + 2h2o which option gives the correct mole ratios? h2s: so2 = 2: 2 and o2: h2o = 3: 2 h2s: so2 = 2: 3 and o2: h2o = 3: 2 h2s: so2 = 4: 4 and o2: h2o = 5: 4 h2s: so2 = 4: 6 and o2: h2o = 4: 4
Answers: 1

Chemistry, 22.06.2019 09:50
Achemist has dissolved a certain substance in water. the chemist knows that more of the substance could be dissolved into the water before it stops dissolving. therefore
Answers: 2

Chemistry, 22.06.2019 21:00
Once similarity and one difference between a mixture of elements and a mixture of compounds
Answers: 3

Chemistry, 22.06.2019 22:30
Consider a culture medium on which only gram-positive organisms such as staphylococcus aureus colonies can grow due to an elevated nacl level. a yellow halo surrounds the growth, indicating the bacterium fermented a sugar in the medium, decreasing the ph as a result and changing the color of a ph indicator chemical. this type of medium would be referred to as a differential and enrichment culture.
Answers: 2
You know the right answer?
Copper exists naturally as two isotopes copper-63 with a mass of 62.93 amu and copper-65 with a mass...
Questions

History, 26.06.2019 05:00





Mathematics, 26.06.2019 05:00

Chemistry, 26.06.2019 05:00

Mathematics, 26.06.2019 05:00

Mathematics, 26.06.2019 05:00

Mathematics, 26.06.2019 05:00





Physics, 26.06.2019 05:00

Mathematics, 26.06.2019 05:00

History, 26.06.2019 05:00

Chemistry, 26.06.2019 05:00


Mathematics, 26.06.2019 05:00

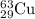 isotope is 69 %.
isotope is 69 %.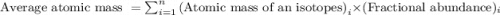 .....(1)
.....(1)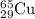 isotope:
isotope:![63.55=[(62.93\times x)+(64.93\times (1-x))]\\\\x=0.69](/tpl/images/0148/0682/8d7a8.png)