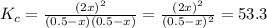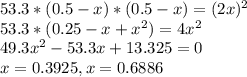Chemistry, 29.07.2019 21:10 sreeranjanig
At a certain temperature, the equilibrium constant, , for this reaction is 53.3. h2(g)+i2(g)↽−−⇀2hi(=53.3 at this temperature, 0.500 mol h2 and 0.500 mol i2 were placed in a 1.00 l container to react. what concentration of hi is present at equilibrium?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:00
Classify each statement about effective nuclear charge, zeff, as true or false.
Answers: 2

Chemistry, 21.06.2019 19:30
The ph of carrots are 5.0 how it is classified a.acidic b.basic c.indicator d.neutral
Answers: 2

Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be changed varied experimented controlled
Answers: 1

Chemistry, 22.06.2019 13:50
How does the motion of particles in a gas change as the gas cools
Answers: 2
You know the right answer?
At a certain temperature, the equilibrium constant, , for this reaction is 53.3. h2(g)+i2(g)↽−−⇀2hi(...
Questions






Business, 24.07.2019 10:00

Spanish, 24.07.2019 10:00

Mathematics, 24.07.2019 10:00

History, 24.07.2019 10:00

Spanish, 24.07.2019 10:00



History, 24.07.2019 10:00







![K_{c}=\frac{[HI]^{2}}{[I_{2}][H_{2}]}](/tpl/images/0148/0680/ff3a2.png)
![[HI]={[HI]}_{0}+2x\\{[H_{2}]}={[H_{2}]}_{0}-x\\{[I_{2}]}={[I_{2}]}_{0}-x\\](/tpl/images/0148/0680/e5b4e.png)