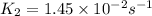Chemistry, 29.07.2019 19:20 faithtunison
The reaction c4h8(g)⟶2c2h4(g) c4h8(g)⟶2c2h4(g) has an activation energy of 262 kj/mol.262 kj/mol. at 600.0 k,600.0 k, the rate constant, , is 6.1×10−8 s−1.6.1×10−8 s−1. what is the value of the rate constant at 785.0 k?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 3

Chemistry, 22.06.2019 10:30
Apiece of metal with a length of 1.42 cm was measured using four different instruments. which of the following measurements is the most accurate?
Answers: 3

Chemistry, 22.06.2019 18:00
Alidded glass container is filled with a colored gas. after a period of time, it is observed that the gas is uniformly spread throughout the box and that the movement has slowed considerably. next, a warm iron plate is carefully placed under the box. why is there resumed movement of the gas in the container?
Answers: 2

Chemistry, 23.06.2019 00:30
The footprints of a dinosaur and the burrow of an ancient shrimp are examples of which kind of fossils
Answers: 2
You know the right answer?
The reaction c4h8(g)⟶2c2h4(g) c4h8(g)⟶2c2h4(g) has an activation energy of 262 kj/mol.262 kj/mol. at...
Questions


English, 18.12.2020 04:40


Business, 18.12.2020 04:40




English, 18.12.2020 04:40

Chemistry, 18.12.2020 04:40






Computers and Technology, 18.12.2020 04:40

English, 18.12.2020 04:40




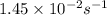
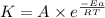
![\log (\frac{K_2}{K_1})=\frac{Ea}{2.303\times R}[\frac{1}{T_1}-\frac{1}{T_2}]](/tpl/images/0147/7591/6d953.png)
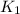 = rate constant at
= rate constant at 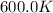 =
= 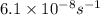
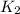 = rate constant at
= rate constant at 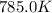 = ?
= ?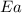 = activation energy for the reaction = 262 kJ/mole = 262000 J/mole
= activation energy for the reaction = 262 kJ/mole = 262000 J/mole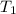 = initial temperature =
= initial temperature = 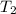 = final temperature =
= final temperature = ![\log (\frac{K_2}{6.1\times 10^{-8}s^{-1}})=\frac{262000J/mole}{2.303\times 8.314J/mole.K}[\frac{1}{600.0K}-\frac{1}{785.0K}]](/tpl/images/0147/7591/a9479.png)