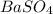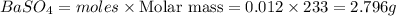Chemistry, 29.07.2019 19:10 crystalclear99
How many grams of barium sulfate can be produced from the reaction of 2.54 grams sodium sulfate and 2.54 g barium chloride? na2so4(aq) + bacl2(aq) --> baso4(s) + 2nacl(aq) report your answer to 3 decimal places.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 13:50
The electron configuration for chromium is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3 d 5 4 s 1 instead of 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3 d 4 4 s 1 . the configuration is an exception to the
Answers: 3

Chemistry, 23.06.2019 00:50
Which of the following warnings would an agricultural chemist tell a farmer who wants to recycle his or her own ammonia? recycling ammonia is a difficult process that sometimes takes weeks. recycling ammonia requires a degree in biochemistry or a related field. recycling ammonia can be harmful because it is highly flammable and toxic. recycling ammonia costs too much money considering the price of the necessary chemicals.
Answers: 1

Chemistry, 23.06.2019 08:00
Drag each pressure unit with the corresponding number to describe standard atmospheric pressure
Answers: 1

Chemistry, 23.06.2019 10:10
Solid tin exists in two forms: white and gray. for the transformation sn(s, white) → sn(s, gray) the enthalpy change is -2.1 kj/mol and the entropy change is -7.4 j/(mol*k). a. calculate the gibbs free energy change for the conversion of 1.00 mol white tin to gray tin at -30℃. b. will white tin convert spontaneously to gray tin at -30℃? c. at what temperature are white and gray tin thermodynamically equivalent at a pressure of 1 atm?
Answers: 3
You know the right answer?
How many grams of barium sulfate can be produced from the reaction of 2.54 grams sodium sulfate and...
Questions



Mathematics, 05.02.2021 20:40

Mathematics, 05.02.2021 20:40



Mathematics, 05.02.2021 20:40


Social Studies, 05.02.2021 20:40


Mathematics, 05.02.2021 20:40


Mathematics, 05.02.2021 20:40

Advanced Placement (AP), 05.02.2021 20:40



Chemistry, 05.02.2021 20:40


Mathematics, 05.02.2021 20:40

Mathematics, 05.02.2021 20:40

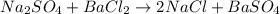
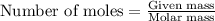
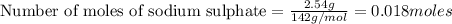
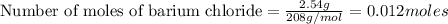
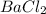 reacts with 1 mole of
reacts with 1 mole of 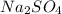
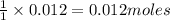 of
of