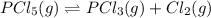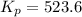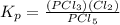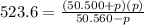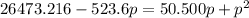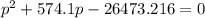Chemistry, 29.07.2019 17:30 davestrider404
For the reaction pcl5(g) 4 pcl3(g) 1 cl2(g) kp 5 23.6 at 500 k a. calculate the equilibrium partial pressures of the reactants and products at 500 k if the initial pressures are ppcl5 5 0.560 atm and ppcl3 5 0.500 atm.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:40
What kind of ion is contained in salts that produce an acidic solution? a positive ion that attracts a proton from water a positive ion that releases a proton to water a negative ion that attracts a proton from water a negative ion that releases a proton to water
Answers: 1

Chemistry, 21.06.2019 20:30
Asample of radium-226 will decay 1/4 of its original amount after 3200years. what is the half-life of radium-226?
Answers: 2

Chemistry, 22.06.2019 02:00
How many moles of magnesium is 3.01 x10^22 atoms of magnesium?
Answers: 1

Chemistry, 22.06.2019 07:50
In which situation can a mixture always be called a solution
Answers: 3
You know the right answer?
For the reaction pcl5(g) 4 pcl3(g) 1 cl2(g) kp 5 23.6 at 500 k a. calculate the equilibrium partial...
Questions

Mathematics, 08.11.2020 23:10



Computers and Technology, 08.11.2020 23:10





Biology, 08.11.2020 23:10

Mathematics, 08.11.2020 23:10


Mathematics, 08.11.2020 23:10


History, 08.11.2020 23:10



Mathematics, 08.11.2020 23:10

Mathematics, 08.11.2020 23:10


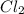 = 42.9 atm,
= 42.9 atm, 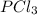 = 93.4 atm and
= 93.4 atm and 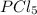 = 7.66 atm
= 7.66 atm