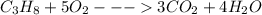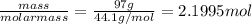He combustion of propane (c3h8) is given by the balanced chemical equation c_3h_8+5o_2\longrightarrow3co_2+4h_ 2o c 3 h 8 + 5 o 2 ⟶ 3 c o 2 + 4 h 2 o how many grams of carbon dioxide gas (co2) are produced burning 97 g of propane? round your answer to the nearest gram.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Acylinder is filled with 2.00 moles of nitrogen, 3.00 moles of argon and 5.00 moles of helium. if the gas mixture is at stp, what is the partial pressure of the argon
Answers: 1


Chemistry, 22.06.2019 06:00
The tilt of the earth's axis of rotation is responsible for the a) ocean's tides. b) size of the moon. c) brightness of stars. d) earth’s seasons.
Answers: 1

Chemistry, 22.06.2019 06:30
If 1.8 l of water is added to 2.5l of a 7.0 molarity koh solution, what is the molarity of the new solution
Answers: 1
You know the right answer?
He combustion of propane (c3h8) is given by the balanced chemical equation c_3h_8+5o_2\longrightarro...
Questions

Mathematics, 08.11.2020 05:30


Chemistry, 08.11.2020 05:30

Physics, 08.11.2020 05:30

English, 08.11.2020 05:30

English, 08.11.2020 05:30




Mathematics, 08.11.2020 05:30



Physics, 08.11.2020 05:30






Social Studies, 08.11.2020 05:30