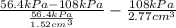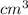Chemistry, 27.07.2019 04:10 winterrs12
The volume of gas at 20°c and 1.00 bar that adsorbed on a cold surface was 1.52 cm3 at 56.4 kpa and 2.77 cm3 at 108 kpa. find the equilibrium constant, and volume of a monolayer.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 09:40
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid synthesis. an industrial chemist studying this reaction fills a 25.0l tank with 4.5 mol of sulfur dioxide gas and 4.5 mol of oxygen gas at 30.°c. he then raises the temperature, and when the mixture has come to equilibrium measures the amount of sulfur trioxide gas to be 1.4 mol. calculate the concentration equilibrium constant for the reaction of sulfur dioxide and oxygen at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 3

Chemistry, 22.06.2019 16:50
Ajet plane is speeding down the runway during takeoff. air resistance is not negligible. identify the forces on the jet.
Answers: 3

Chemistry, 23.06.2019 04:10
What does the field of thermodynamics relate to a-changes in nuclear reactions b- changes in energy in systems c changes in molecular structure d changes in atomic properties
Answers: 1
You know the right answer?
The volume of gas at 20°c and 1.00 bar that adsorbed on a cold surface was 1.52 cm3 at 56.4 kpa and...
Questions

Mathematics, 15.12.2020 20:40

Health, 15.12.2020 20:40


Mathematics, 15.12.2020 20:40

Mathematics, 15.12.2020 20:40

Mathematics, 15.12.2020 20:40


Advanced Placement (AP), 15.12.2020 20:40


Mathematics, 15.12.2020 20:40

Mathematics, 15.12.2020 20:40

Mathematics, 15.12.2020 20:40


Mathematics, 15.12.2020 20:40

Mathematics, 15.12.2020 20:40

Spanish, 15.12.2020 20:40

Mathematics, 15.12.2020 20:40

Mathematics, 15.12.2020 20:40


Mathematics, 15.12.2020 20:40

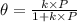
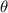 = fraction coverage by gas molecules
= fraction coverage by gas molecules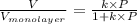
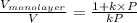
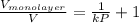
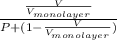
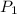 and
and 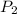 for
for 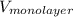 as follows.
as follows.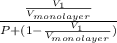 =
= 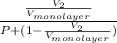
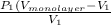 =
= 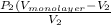
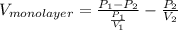 .......... (1)
.......... (1)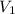 is
is 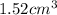 and
and 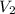 is
is 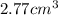 .
.