
Chemistry, 27.07.2019 03:20 glowbaby123
A25 -ml of 0.30 m of ammonia, nh (a), solution is titrated with 0.15 m hydrochloric acid, hcl (aq calculate the ph of the solution a) determine the volume of the acid required to reach equivalence point. b) at half the equivalence point. c) at the cquivalence point. d) when 55 ml of the hcl has been added. e choose a suitable indicator for this titration.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:30
150.0 grams of asf3 were reacted with 180.0 g of ccl4 to produce ascl3 and ccl2f2. if the actual yield of ccl2f2 was 127 g, what is the percent yield?
Answers: 2

Chemistry, 22.06.2019 01:30
What is the value of keq for the reaction expressed in scientific notation
Answers: 1

Chemistry, 22.06.2019 08:00
Identify a strong intermolecular force of attraction between an alcohol
Answers: 1

Chemistry, 22.06.2019 12:00
What is the percentage of hydrogen in nitrogen trihydride
Answers: 1
You know the right answer?
A25 -ml of 0.30 m of ammonia, nh (a), solution is titrated with 0.15 m hydrochloric acid, hcl (aq ca...
Questions



English, 27.09.2020 19:01

English, 27.09.2020 19:01


History, 27.09.2020 19:01


History, 27.09.2020 19:01

Mathematics, 27.09.2020 19:01







Mathematics, 27.09.2020 19:01

English, 27.09.2020 19:01

Mathematics, 27.09.2020 19:01

History, 27.09.2020 19:01

Medicine, 27.09.2020 19:01

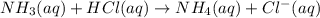
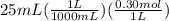
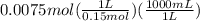
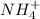 will form and the solution will act as a buffer solution as it has a weak base(ammonia) and its conjugate acid(ammonium ion).
will form and the solution will act as a buffer solution as it has a weak base(ammonia) and its conjugate acid(ammonium ion).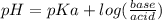
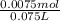
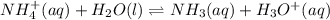
![Ka=\frac{[x][x]}{[0.10-x]}](/tpl/images/0137/3088/1f1fd.png)
![Ka=\frac{[x]^2}{[0.10-x]}Ka for [tex]NH_4^+](/tpl/images/0137/3088/dc53e.png) is
is 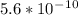 . It's a very low value so the x on the bottom could be neglected and the expression could be written as:
. It's a very low value so the x on the bottom could be neglected and the expression could be written as:
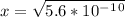
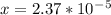
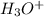 is
is 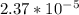 M.
M. 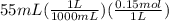
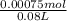 = 0.009375 M
= 0.009375 M

