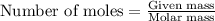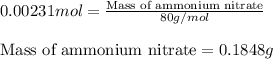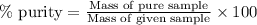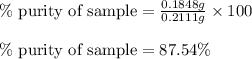Chemistry, 27.07.2019 03:10 JvGaming2001
Ammonium nitrate (nh4n03) is one of the most important nitrogen-containing fertilizers. its purity can be analyzed by titrating a solution of nh4no3 with a standard naoh solution. in one experiment a 0.2111-g sample of industrially prepared nh4no3 required 22.62 ml of 0.1023 m naoh for neutralization (a) enter a net ionic equation for the reaction (include states of matter) (b) what is the percent purity of the sample?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Select all that apply. a beta particle: is electromagnetic energy is an electron has zero charge is emitted from the nucleus has a +2 charge has a -1 charge
Answers: 1

Chemistry, 22.06.2019 04:30
Suppose that during that icy hot lab 65,000 j of energy were transferred to 450 g of water at 20°c what would have have been the final temperature of the water
Answers: 2

Chemistry, 22.06.2019 07:00
The organism shown is a free-living one that is anchored to the bottom of ponds and streams during one stage of its life cycle what is the common name for the group to which this organism belong
Answers: 3

Chemistry, 22.06.2019 15:30
The gulf stream is a warm water current that flows away from the equator to northern europe. witch of these does it cause. a. crashes of warm and cool water in the ocean b.colder climates near the equator c.large waves on the cost of europe d.warm climates in northern europe
Answers: 1
You know the right answer?
Ammonium nitrate (nh4n03) is one of the most important nitrogen-containing fertilizers. its purity c...
Questions



English, 12.06.2021 21:10

Mathematics, 12.06.2021 21:10

English, 12.06.2021 21:10

Social Studies, 12.06.2021 21:10

SAT, 12.06.2021 21:10


Social Studies, 12.06.2021 21:10




English, 12.06.2021 21:10

Chemistry, 12.06.2021 21:20

Mathematics, 12.06.2021 21:20





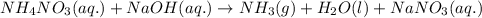
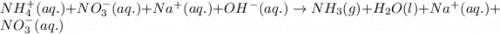
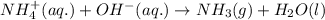
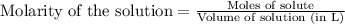
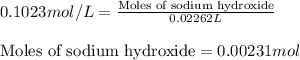
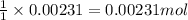 of ammonium nitrate.
of ammonium nitrate.