
Chemistry, 27.07.2019 02:30 Rockey3876
How many grams of ice would be melted by the energy obtained as 16.1 g of steam is condensed at 100°c and cooled to 0°c? specific heat (ice) = 2.10 j/gºc specific heat (water) = 4.18 j/gºc heat of fusion = 333 j/g heat of vaporization = 2258 j/g 43.1 kg 36.4 kg 129 g 6.73 kg 20 g

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 13:30
Mary is conducting an experiment on how pollution affects plant growth. how can she ensure that her data are reliable?
Answers: 3

Chemistry, 22.06.2019 14:10
16. in a reaction that has reached equilibrium, a. the forward and reverse reactions are occurring at the same rate. b. the reactants and products are in equal concentrations. c. the forward reaction has gone further than the reverse reaction. d. there are equal numbers of atoms on both sides of the equation. e. a, b, and d are correct.
Answers: 2

Chemistry, 23.06.2019 01:30
In what way do investigations build scientific knowledge? the results of investigations lead to questions that cannot be tested. they reflect the opinions and social values of scientists, ensuring valid information. the results of investigations lead to new questions, which lead to new investigations. they are not influenced by the research of earlier scientists, so they are able to address gaps in understanding.i
Answers: 1

Chemistry, 23.06.2019 08:00
If the solubility of a gas in water is 1.22 g/l at 2.75 atm, what is its solubility (in g/l) at 1.0 atm?
Answers: 1
You know the right answer?
How many grams of ice would be melted by the energy obtained as 16.1 g of steam is condensed at 100°...
Questions


Mathematics, 09.02.2021 04:20


Mathematics, 09.02.2021 04:20

Mathematics, 09.02.2021 04:20






Mathematics, 09.02.2021 04:20




Mathematics, 09.02.2021 04:20





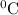
![[16.1\times 2258]J+[16.1\times 4.18\times (100-0)]J](/tpl/images/0137/1545/534f5.png) = 43083.6 J
= 43083.6 J



