
Chemistry, 27.07.2019 01:10 lillianrhoades2
Menthol, the substance we can smell in mentholated cough drops, is composed of c, h, and o. a 0.1005 g sample of menthol is combusted, producing 0.2829 g of carbon dioxide and 0.1159 g of water. what is the empirical formula for menthol? if mentho has a molar mass of 156 g/mol, what is its molecular formula?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:40
During which time interval does the object travel approximately 10 meters
Answers: 3

Chemistry, 22.06.2019 02:20
Calculate the molarity of 48.0 ml of 6.00 m h2so4 diluted to 0.250 l .
Answers: 1

Chemistry, 22.06.2019 17:40
How much heat is added if 0.814g of water increase in temperature by 0.351 degree c?
Answers: 3

Chemistry, 22.06.2019 23:00
What is the oxidation state of each individual carbon atom in c2o42−?
Answers: 1
You know the right answer?
Menthol, the substance we can smell in mentholated cough drops, is composed of c, h, and o. a 0.1005...
Questions








Mathematics, 12.10.2020 23:01

Chemistry, 12.10.2020 23:01

Mathematics, 12.10.2020 23:01

Mathematics, 12.10.2020 23:01


Mathematics, 12.10.2020 23:01


Mathematics, 12.10.2020 23:01


Mathematics, 12.10.2020 23:01


Mathematics, 12.10.2020 23:01

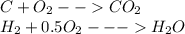
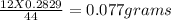 of carbon
of carbon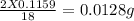 hydrogen
hydrogen

