Calculate the net change in energy for the following reaction:
2na(s) + 2hcl(g) → 2nacl(s) +...

Chemistry, 26.07.2019 16:20 flowersthomas1969
Calculate the net change in energy for the following reaction:
2na(s) + 2hcl(g) → 2nacl(s) + h2(g)
given the following information:
energy of sublimation of na(s) = 97 kj/mol
bond energy of hcl = 427 kj/mol
ionization energy of na(g) = 496 kj/mol
electron affinity of cl(g) = –349 kj/mol
lattice energy of nacl(s) = –778 kj/mol
bond energy of h2 = 432 kj/mol

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 08:30
Which metal exist in liquid state and can be cut with knife ?
Answers: 2

Chemistry, 22.06.2019 09:00
Chen drew a diagram to compare the ways in which different organisms obtain nitrogen. which label belongs to the area marked z?
Answers: 3

You know the right answer?
Questions

Mathematics, 13.11.2020 22:50


Chemistry, 13.11.2020 22:50


History, 13.11.2020 22:50


Mathematics, 13.11.2020 22:50


Mathematics, 13.11.2020 22:50

Mathematics, 13.11.2020 22:50



Mathematics, 13.11.2020 22:50




Mathematics, 13.11.2020 22:50

Biology, 13.11.2020 22:50

Biology, 13.11.2020 22:50

History, 13.11.2020 22:50

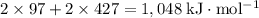 .
.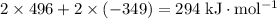 .
.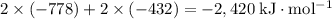 .
.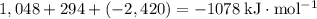 .
.

