
In the first step of the ostwald process for the synthesis of nitric acid, ammonia is converted to nitric oxide by the high-temperature reaction 4 nh31g2 + 5 o21g2 ¡ 4 no1g2 + 6 h2o1g2 (a) how is the rate of consumption of o2 related to the rate of consumption of nh3? (b) how are the rates of formation of no and h2o related to the rate of consumption of nh3

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 17:00
The msds for glacial acetic acid says that it is a flammable liquid that can severely burn any human tissue it comes in contact with. it reacts with bases, various metals, and strong oxidizing agents. its vapors can form explosive mixtures with air.
Answers: 1


Chemistry, 22.06.2019 22:30
Draw the aromatic compound toluene (methylbenzene). show all hydrogen atoms, including those on the ring.
Answers: 1

Chemistry, 23.06.2019 00:00
How do you determine the percent yield of a chemical reaction
Answers: 1
You know the right answer?
In the first step of the ostwald process for the synthesis of nitric acid, ammonia is converted to n...
Questions



English, 11.10.2021 22:30

Mathematics, 11.10.2021 22:30

Mathematics, 11.10.2021 22:30

English, 11.10.2021 22:30




World Languages, 11.10.2021 22:30



Computers and Technology, 11.10.2021 22:30

Mathematics, 11.10.2021 22:30


English, 11.10.2021 22:30

English, 11.10.2021 22:30

Chemistry, 11.10.2021 22:30

Mathematics, 11.10.2021 22:30

Mathematics, 11.10.2021 22:30

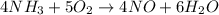
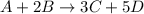
![rate=-\frac{\Delta [A]}{\Delta t}=-\frac{1}{2}\frac{\Delta [B]}{\Delta t}=\frac{1}{3}\frac{\Delta [C]}{\Delta t}=\frac{1}{5}\frac{\Delta [D]}{\Delta t}](/tpl/images/0133/4682/3644a.png)
![rate=-\frac{1}{4}\frac{\Delta [NH_3]}{\Delta t}=-\frac{1}{5}\frac{\Delta [O_2]}{\Delta t}=\frac{1}{4}\frac{\Delta [NO]}{\Delta t}=\frac{1}{6}\frac{\Delta [H_2O]}{\Delta t}](/tpl/images/0133/4682/51a20.png)
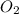 related to rate of consumption of
related to rate of consumption of 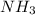 as:
as:![-\frac{1}{5}\frac{\Delta [O_2]}{\Delta t}=-\frac{1}{4}\frac{\Delta [NH_3]}{\Delta t}](/tpl/images/0133/4682/1a857.png)
![\frac{\Delta [O_2]}{\Delta t}=\frac{5}{4}\frac{\Delta [NH_3]}{\Delta t}](/tpl/images/0133/4682/ce2db.png)
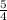 the rate of consumption of ammonia.
the rate of consumption of ammonia.![\frac{1}{4}\frac{\Delta [NO]}{\Delta t}=-\frac{1}{4}\frac{\Delta [NH_3]}{\Delta t}](/tpl/images/0133/4682/91a55.png)
![\frac{\Delta [NO]}{\Delta t}=-\frac{\Delta [NH_3]}{\Delta t}](/tpl/images/0133/4682/c5068.png)
 to the rate of consumption of ammonia would be:
to the rate of consumption of ammonia would be:![\frac{1}{6}\frac{\Delta [H_2O]}{\Delta t}=-\frac{1}{4}\frac{\Delta [NH_3]}{\Delta t}](/tpl/images/0133/4682/41d66.png)
![\frac{\Delta [H_2O]}{\Delta t}=-\frac{6}{4}\frac{\Delta [NH_3]}{\Delta t}](/tpl/images/0133/4682/66644.png)
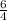 times that is 1.5 times to the rate of consumption of ammonia.
times that is 1.5 times to the rate of consumption of ammonia. 

