
Chemistry, 26.07.2019 03:10 babygirl123468
At 40.8c the value of kw is 2.92 3 10214. a. calculate the [h1] and [oh2] in pure water at 40.8c. b. what is the ph of pure water at 40.8c? c. if the hydroxide ion concentration in a solution is 0.10 m, what is the ph at 40.8c?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:50
An aqueous solution of potassium hydroxide is standardized by titration with a 0.194 m solution of hydrobromic acid. if 25.2 ml of base are required to neutralize 24.2 ml of the acid, what is the molarity of the potassium hydroxide solution? m potassium hydroxide
Answers: 2

Chemistry, 22.06.2019 14:00
Displacement is the slope of a velocity vs. time graph a. true b. false
Answers: 1

Chemistry, 22.06.2019 16:50
Answer asap need it by wednesday morning calculate the ph of 0.02m hcl best answer will be brainliest
Answers: 1

Chemistry, 22.06.2019 17:30
To find the enthalpy of a reaction in the lab, you measured the of the reactants and the change during the reaction.
Answers: 1
You know the right answer?
At 40.8c the value of kw is 2.92 3 10214. a. calculate the [h1] and [oh2] in pure water at 40.8c. b....
Questions

Mathematics, 04.11.2020 01:00

Mathematics, 04.11.2020 01:00


Mathematics, 04.11.2020 01:00







Mathematics, 04.11.2020 01:00

Mathematics, 04.11.2020 01:00



Computers and Technology, 04.11.2020 01:00

Chemistry, 04.11.2020 01:00

Biology, 04.11.2020 01:00

Mathematics, 04.11.2020 01:00


 .
.![K_w=[H^+][OH^-]](/tpl/images/0133/4562/bc68a.png)

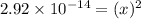

![pH=-\log [H^+]](/tpl/images/0133/4562/37e81.png)


![pOH=-\log [OH^-]](/tpl/images/0133/4562/1fac1.png)





