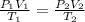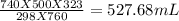Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Write a net ionic equation to show that hydrofluoric acid, behaves as an acid in water.
Answers: 1

Chemistry, 22.06.2019 00:00
Alarge marble is dropped in a graduated cylinder with 35ml of water in it.the water level increases to 49ml.what is the volume of the marble
Answers: 1

Chemistry, 22.06.2019 02:10
26. of of (aq) by (aq) is . if 50.00 ml of 1.05 m is to 25.00 ml of 1.86 m ,at be? ( no is toina of aof) , h.. (p. ). . .
Answers: 3

Chemistry, 22.06.2019 10:20
In a reaction equation, where are the products located? a.) above the arrow b.) to the right of the arrow c.) to the left of the arrow d.) below the arrow
Answers: 2
You know the right answer?
Asample of nitrogen gas had a volume of 500. ml, a pressure in its closed container of 740 torr, and...
Questions




English, 10.05.2021 22:40

Mathematics, 10.05.2021 22:40


Chemistry, 10.05.2021 22:40

Social Studies, 10.05.2021 22:40


Mathematics, 10.05.2021 22:40







Mathematics, 10.05.2021 22:40



Mathematics, 10.05.2021 22:40