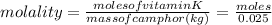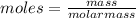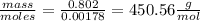Chemistry, 25.07.2019 21:20 Natasha019
Vitamin k is involved in normal blood clotting. when 0.802 g of vitamin k is dissolved in 25.0 g of camphor, the freezing point of the solution is lowered by 2.69 °c. the freezing point and kf constant for camphor can be found here. calculate the molar mass of vitamin k.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Why are people not able to scuba dive in the deep part of the ocean
Answers: 2

Chemistry, 22.06.2019 07:30
Compare and contrast the bohr model and the electron cloud models of the atom.
Answers: 1

Chemistry, 22.06.2019 10:00
How many mmols of tris-hcl are there in 100 ml of a 100 mm tris-hcl buffer solution at ph 8.1? note that the 100 mm refers to the sum of tris and tris-hcl concentrations?
Answers: 3

Chemistry, 22.06.2019 11:30
Aperfume bottle is dropped in the corner of a room. the odor of the perfume can be detected on the other side of the room. which statement best describes this observation?
Answers: 2
You know the right answer?
Vitamin k is involved in normal blood clotting. when 0.802 g of vitamin k is dissolved in 25.0 g of...
Questions

Biology, 01.04.2021 19:00




Chemistry, 01.04.2021 19:00

Mathematics, 01.04.2021 19:00







Geography, 01.04.2021 19:00



Mathematics, 01.04.2021 19:00



Mathematics, 01.04.2021 19:00

Mathematics, 01.04.2021 19:00