Chemistry, 25.07.2019 20:20 NatalieAllen11
Enter your answer in the provided box. pentaborane−9 (b5h9) is a colorless, highly reactive liquid that will burst into flames when exposed to oxygen. the reaction is 2b5h9(l) 12o2(g) → 5b2o3(s) 9h2o(l) calculate the kilojoules of heat released per gram of the compound reacted with oxygen. the standard enthalpy of formations of b5h9(l), b2o3(s), and h2o(l) are 73.2, −1271.94, and −285.83 kj/mol, respectively.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 14:30
Select the word from the list that best fits the definition the nuclear family into which a person is born or adopted.
Answers: 2

Chemistry, 23.06.2019 04:00
Achemical reaction is done in the setup shown , resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 2

Chemistry, 23.06.2019 11:30
Which of these have the same number of particles as 1 mole of water h2o
Answers: 1

Chemistry, 23.06.2019 15:30
Dona wrote the characteristics of two types of galaxies as shown below: type a: has a large flattened core type b: does not have a regular shape which statement is correct? type a is an irregular galaxy and type b is a lens galaxy. type a is a lens galaxy and type b is an irregular galaxy. type a is a spiral galaxy and type b is an elliptical galaxy. type a is an elliptical galaxy and type b is a spiral galaxy.
Answers: 2
You know the right answer?
Enter your answer in the provided box. pentaborane−9 (b5h9) is a colorless, highly reactive liquid t...
Questions



Biology, 09.10.2019 10:30


English, 09.10.2019 10:30

Mathematics, 09.10.2019 10:30



Biology, 09.10.2019 10:30

Mathematics, 09.10.2019 10:30




English, 09.10.2019 10:30

English, 09.10.2019 10:30



History, 09.10.2019 10:30

Mathematics, 09.10.2019 10:30

Computers and Technology, 09.10.2019 10:30

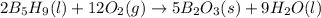
![\Delta H=\sum [n\times \Delta H_f(product)]-\sum [n\times \Delta H_f(reactant)]](/tpl/images/0132/3420/76c37.png)
![\Delta H=[(n_{H_2O}\times \Delta H_{H_2O})+(n_{B_2O_3}\times \Delta H_{B_2O_3})]-[(n_{B_5H_9}\times \Delta H_{B_5H_9})+(n_{O_2}\times \Delta H_{O_2})]](/tpl/images/0132/3420/48532.png)
![\Delta H=[(9\times -285.83)+(5\times -1271.94)]-[(2\times 73.2)+(12\times 0)]\\\\\Delta H=-9078.57kJ](/tpl/images/0132/3420/9a745.png)
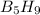 has 63.12 grams of mass
has 63.12 grams of mass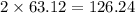 grams of mass
grams of mass