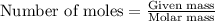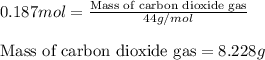Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:40
Write the formula for the following chemicals. 7. e. trinitrogen tetraoxide a calcium phosphate f. magnesium acetate b. potassium sulfide g nickel(iii) cyanide c carbon dioxide h. silver sulfate d. cobalt(ii) chloride
Answers: 1

Chemistry, 22.06.2019 10:00
Which sentence about particles in matter is true? a. atoms are present in solids and liquids but not in gases. b. the particles of matter are in constant motion. c. the same kinds of atoms are found in different elements. d. when a solid changes to a liquid, the sizes of the particles change.
Answers: 1

Chemistry, 22.06.2019 10:20
In a reaction equation, where are the products located? a.) above the arrow b.) to the right of the arrow c.) to the left of the arrow d.) below the arrow
Answers: 2

Chemistry, 23.06.2019 07:30
Chris is about to do an experiment to measure the density of water at several temperatures. his teacher has him prepare and sign a safety contract before beginning the experiment. which term is mostlikely part of the safety contract
Answers: 3
You know the right answer?
Determine the mass of co2 produced by burning enough of methane to produce 1.50×102kj of heat. ch4(g...
Questions

Mathematics, 21.10.2020 18:01

Geography, 21.10.2020 18:01


History, 21.10.2020 18:01


Mathematics, 21.10.2020 18:01

History, 21.10.2020 18:01

Mathematics, 21.10.2020 18:01

English, 21.10.2020 18:01



Mathematics, 21.10.2020 18:01


Biology, 21.10.2020 18:01

Biology, 21.10.2020 18:01

Spanish, 21.10.2020 18:01

English, 21.10.2020 18:01



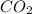 produced will be 8.228 g.
produced will be 8.228 g.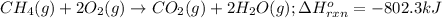
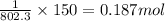 of carbon dioxide is produced.
of carbon dioxide is produced.