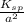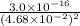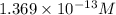Chemistry, 25.07.2019 04:30 wwesuplexcity28
Determine the molar solubility ( ) of zn(cn)2 in a solution with a ph=1.33 . ignore activities. the for zn(cn)2 is 3.0×10−16 . the for hcn is 6.2×10−10 .

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:40
What is the value of keq for the reaction expressed in scientific notation?
Answers: 1

Chemistry, 22.06.2019 09:10
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 09:30
What are scientists who study fossils called? ( a ) astronomers. ( b ) biologists. ( c ) geologists. ( d ) paleontologists.
Answers: 2

You know the right answer?
Determine the molar solubility ( ) of zn(cn)2 in a solution with a ph=1.33 . ignore activities. the...
Questions

Mathematics, 29.10.2019 00:31


Social Studies, 29.10.2019 00:31

Mathematics, 29.10.2019 00:31

Social Studies, 29.10.2019 00:31

Mathematics, 29.10.2019 00:31



Mathematics, 29.10.2019 00:31




Mathematics, 29.10.2019 00:31

Social Studies, 29.10.2019 00:31


English, 29.10.2019 00:31

Geography, 29.10.2019 00:31


Mathematics, 29.10.2019 00:31

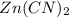 in the given solution.
in the given solution.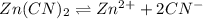
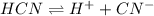
![[H^{+}]](/tpl/images/0129/7944/85507.png)
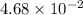
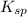 =
= ![[Zn^{2+}][CN^{-}]^{2}](/tpl/images/0129/7944/9ff82.png)
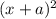
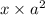 ( x + a = a because a >> x)
( x + a = a because a >> x)