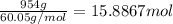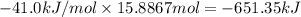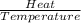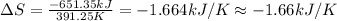Chemistry, 25.07.2019 04:20 thawkins79
The heat of vaporization δhv of acetic acid hch3co2 is 41.0 /kjmol. calculate the change in entropy δs when 954.g of acetic acid condenses at 118.1°c. be sure your answer contains a unit symbol. round your answer to 3 significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Ionic compounds are made of ions, and yet the overall charge of an ionic compound is neutral. why?
Answers: 1

Chemistry, 22.06.2019 13:50
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3

Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 2

Chemistry, 22.06.2019 18:00
Chlorophyll a had the molecular formula c55h72mgn4o5 how many atoms are in this molecule
Answers: 2
You know the right answer?
The heat of vaporization δhv of acetic acid hch3co2 is 41.0 /kjmol. calculate the change in entropy...
Questions

Geography, 23.04.2020 19:02




Mathematics, 23.04.2020 19:02

Mathematics, 23.04.2020 19:02

French, 23.04.2020 19:02



Mathematics, 23.04.2020 19:02

Mathematics, 23.04.2020 19:02


Business, 23.04.2020 19:02



Mathematics, 23.04.2020 19:02

Mathematics, 23.04.2020 19:02

Chemistry, 23.04.2020 19:02