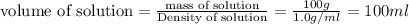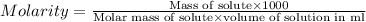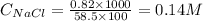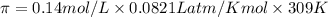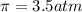Chemistry, 25.07.2019 04:10 connorvoss5805
At 36°c, what is the osmotic pressure of a 0.82% nacl by weight aqueous solution? assume the density of the solution is 1.0 g/ml. (r = 0.0821 l · atm/(k · mol)) a. 7.1 atm b. 0.35 atm c. 0.82 atm d. 4.1 × 102 atm e. 3.5 atm

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 08:00
Define dew point. i am writing this part to be able to ask the question
Answers: 1

Chemistry, 22.06.2019 12:00
Ineed this asap part i: scientific method what is the difference between science and pseudoscience? what is the scientific method?
Answers: 2

Chemistry, 22.06.2019 14:00
Will mark brainliest how many electrons can be held in the energy level n = 4?
Answers: 1
You know the right answer?
At 36°c, what is the osmotic pressure of a 0.82% nacl by weight aqueous solution? assume the densit...
Questions

History, 20.07.2019 14:30


Biology, 20.07.2019 14:30


















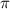 = osmotic pressure = ?
= osmotic pressure = ?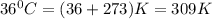
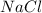 is dissolved in 100 g of solution.
is dissolved in 100 g of solution.