

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 18:30
When a device is used in a circuit in which the voltage is 81 v the current flowing through the device is 3 a what is the resistance of the device
Answers: 2

Chemistry, 22.06.2019 18:30
Asample of hydrated tin (ii) chloride (sncl2) has a mass of 4.90 g. when it is dehydrated, it has a mass of 4.10 g. which is the correct chemical formula for the hydrate? sncl2•2h2o sncl2•4h2o sncl2•6h2o
Answers: 2

You know the right answer?
Consider the neutralization reaction 2hno3(aq)+ba(oh)2(aq)⟶2h2o(l)+ba(no 3)2(aq) a 0.105 l sample of...
Questions






Mathematics, 22.06.2019 23:00

Mathematics, 22.06.2019 23:00


Mathematics, 22.06.2019 23:00


Mathematics, 22.06.2019 23:00

Mathematics, 22.06.2019 23:00


Computers and Technology, 22.06.2019 23:00





Chemistry, 22.06.2019 23:00

Biology, 22.06.2019 23:00

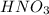 comes out to be 0.088 M.
comes out to be 0.088 M.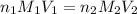
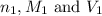 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 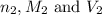 are the n-factor, molarity and volume of base which is
are the n-factor, molarity and volume of base which is 




