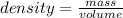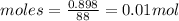Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 15:20
Which description best characterizes the motion of particles in a solid?
Answers: 2


Chemistry, 22.06.2019 21:30
Plzz a sample of table sugar (sucrose, c12h22o11) has a mass of 7.801 g. ● a) calculate the number of moles of c12h22o11 in the sample b) calculate the number of moles of each element in c12h22o11 (number of moles of c, number of moles of h & number of moles of o) in the sample. (use your answer from part a as your starting point.) show your work and highlight your final answer. calculate the number of atoms of each element in c12h22o11 (number of atoms of c, number of atoms of h & number of atoms of o) in the sample. (use your answers from part b as your starting for each element.) show your work and highlight your final answer.
Answers: 1

Chemistry, 23.06.2019 04:31
What are the coefficients that will balance the skeleton equation below? n2 + h2 → nh3
Answers: 1
You know the right answer?
1. what volume of toluene do you need to add to 1 ml of ethyl acetate to make an equi-molar mixture?...
Questions

Mathematics, 23.09.2019 00:10

Biology, 23.09.2019 00:10


Arts, 23.09.2019 00:10






Mathematics, 23.09.2019 00:10

Mathematics, 23.09.2019 00:10


Biology, 23.09.2019 00:10


Mathematics, 23.09.2019 00:10


Chemistry, 23.09.2019 00:10

Mathematics, 23.09.2019 00:10

Mathematics, 23.09.2019 00:10