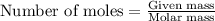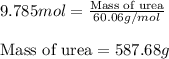Chemistry, 25.07.2019 01:20 1233042260
The emission of no2 by fossil fuel combustion can be prevented by injecting gaseous urea into the combustion mixture. the urea reduces no (which oxidizes in air to form no2) according to the following reaction: 2co(nh2)2(g)+4no(g)+o2(g)→4n2(g)+2c o2(g)+4h2o(g) suppose that the exhaust stream of an automobile has a flow rate of 2.32 l/s at 670 k and contains a partial pressure of no of 12.1 torr. what total mass (in gram) of urea is necessary to react completely with the no formed during 8.1 hours of driving?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:00
In the following redox reaction which is the oxidizing agent and which is the reducing agent? alcl3 + na nacl + al
Answers: 1

Chemistry, 22.06.2019 08:20
What is the formula for the compound dinitrogen pentoxide? a. n4o5 b. n5o4 c. n4o6 d. n5o2 e. n2o5
Answers: 3

Chemistry, 22.06.2019 10:30
Consider the following reactions. (note: (s) = solid, (l) = liquid, and (g) = gas.) mg(s) + ½o2(g) → mgo(s) + 146 kcal/mole h2(g) + ½o2(g) → h2o(g), δh = -57.82 kcal/mole what type of reaction is represented by the previous two examples?
Answers: 3

Chemistry, 22.06.2019 11:40
Consider this equilibrium: n29) + o2(g) + 2no(c).nitrogen gas and oxygen gas react when placed in a closed container. as the reaction proceeds towards equilibrium, what happens to the rate of thereverse reaction?
Answers: 1
You know the right answer?
The emission of no2 by fossil fuel combustion can be prevented by injecting gaseous urea into the co...
Questions

English, 02.08.2019 23:40

History, 02.08.2019 23:40

History, 02.08.2019 23:40

Health, 02.08.2019 23:40





Mathematics, 02.08.2019 23:40

Mathematics, 02.08.2019 23:40


Business, 02.08.2019 23:40


English, 02.08.2019 23:40


Chemistry, 02.08.2019 23:40



Social Studies, 02.08.2019 23:40

History, 02.08.2019 23:40

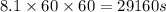
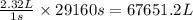
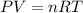
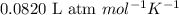
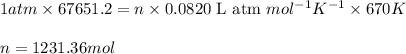
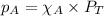
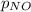 = partial pressure of NO = 12.1 torr
= partial pressure of NO = 12.1 torr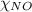 = mole fraction of NO = ?
= mole fraction of NO = ?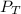 = total pressure of solution = 1 atm = 760 torr (Conversion factor: 1 atm = 760 torr)
= total pressure of solution = 1 atm = 760 torr (Conversion factor: 1 atm = 760 torr)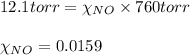
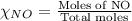
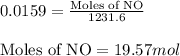
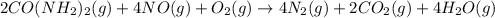
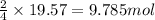 of urea.
of urea.