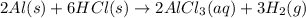Write an equation for the reaction of solid aluminum metal with hydrochloric acid (hydrogen monochloride) dissolved in water to form aluminum chloride dissolved in water and hydrogen gas. when balanced, what is the coefficient for the hydrochloric acid (hydrogen monochloride)?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Agas in a balloon at constant pressure has a volume of 160 ml at -125*c. what is its volume at 29.0*c?
Answers: 1

Chemistry, 22.06.2019 05:40
Consider the elements bromine and chlorine; which elements has a larger ionic radius ?
Answers: 1

Chemistry, 22.06.2019 12:00
Explain what happens at the saturation point when adding salt to water at room temperature.
Answers: 1

Chemistry, 22.06.2019 17:10
)benzene and toluene form nearly ideal solutions. consider an equimolar solution of benzene and toluene. at 20 °c the vapour pressures of pure benzene and toluene are 9.9 kpa and 2.9 kpa, respectively. the solution is boiled by reducing the external pressure below the vapour pressure. calculate (i) the pressure when boiling begins, (ii) the composition of each component in the vapour, and (iii) the vapour pressure when only a few drops of liquid remain. assume that the rate of vaporization is low enough for the temperature to remain constant at 20 °c.
Answers: 1
You know the right answer?
Write an equation for the reaction of solid aluminum metal with hydrochloric acid (hydrogen monochlo...
Questions




History, 23.03.2021 14:00

History, 23.03.2021 14:00

Mathematics, 23.03.2021 14:00




Mathematics, 23.03.2021 14:00

Mathematics, 23.03.2021 14:00


Mathematics, 23.03.2021 14:00


Mathematics, 23.03.2021 14:00

Mathematics, 23.03.2021 14:00

History, 23.03.2021 14:00


Chemistry, 23.03.2021 14:00

Chemistry, 23.03.2021 14:00