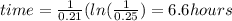Chemistry, 24.07.2019 22:30 adejumoayobami1
The radioactive isotope of lead, pb-209, decays at a rate proportional to the amount present at time t and has a half-life of 3.3 hours. if 1 gram of this isotope is present initially, how long will it take for 75% of the lead to decay? (round your answer to two decimal places.)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:30
The chart shows the bid provided by four contractors to complete a job. which contractor is the most cost-effective?
Answers: 3

Chemistry, 22.06.2019 21:30
In science class richard learns that a substance has a boiling point of 230 fahrenheit his teacher ask him to convert this temperature to degrees celsius what is the boiling point of his substance in degrees celsius
Answers: 3

Chemistry, 22.06.2019 21:50
What is a main difference between a mixture and a pure substance? a mixture is only a liquid, but a pure substance can be in any state.a mixture looks the same throughout, but a pure substance does not.1 a mixture can vary in composition, but a pure substance has a set composlo a mixture can be made up of a single compound, but a pure substance car
Answers: 2

Chemistry, 23.06.2019 07:10
1) a light bulb takes in 30 of energy per second. it transfers 3j as use energy. calculate the efficiency. second. it transfers 3j as useful light energy and 27j as heat energy. calculate the efficiency
Answers: 1
You know the right answer?
The radioactive isotope of lead, pb-209, decays at a rate proportional to the amount present at time...
Questions


















Chemistry, 26.10.2019 03:43


Biology, 26.10.2019 03:43

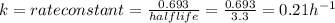
![time=\frac{1}{k}(ln[\frac{A_{0}}{A_{t}}]](/tpl/images/0128/8405/a5dc9.png)