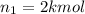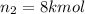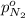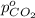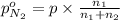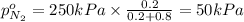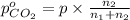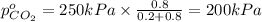Chemistry, 24.07.2019 19:10 holasoykawaii10
Arigid tank is divided into two equal volumes. one side contains 2 kmol of nitrogen n2 at 500 kpa while the other side contains 8 kmol of co2 at 200 kpa. the two sides are now connected and the gases are mixed and forming a homogeneous mixture at 250 kpa. find the partial pressure of the co2 in the final mixture.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:00
What were the success and failures that came to boyle’s excitements?
Answers: 1

Chemistry, 22.06.2019 05:00
Agas can holds 2.0 gal of gasoline. what is this quantity in cubic centimeters?
Answers: 2

Chemistry, 22.06.2019 08:50
If two atoms are bonded to a central atom with no lone pairs,how will they be arranged
Answers: 3

Chemistry, 22.06.2019 13:00
How many moles of sulfur dioxide are produced when 4.38 moles of oxygen completely react with iron (iv) sulfide
Answers: 2
You know the right answer?
Arigid tank is divided into two equal volumes. one side contains 2 kmol of nitrogen n2 at 500 kpa wh...
Questions


Computers and Technology, 26.07.2019 00:00





Advanced Placement (AP), 26.07.2019 00:00

History, 26.07.2019 00:00







Physics, 26.07.2019 00:00



Physics, 26.07.2019 00:00

English, 26.07.2019 00:00

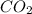 in the final mixture is 200 kPa.
in the final mixture is 200 kPa.