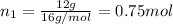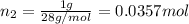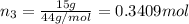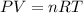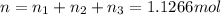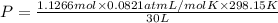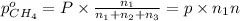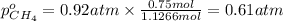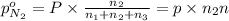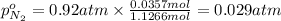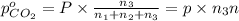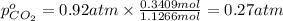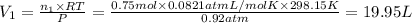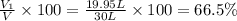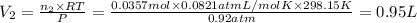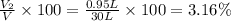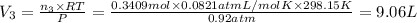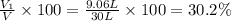Chemistry, 24.07.2019 16:10 jacksonhoyt8049
A30-liter volume of gas at 25°c contains 12 g of methane, 1 g of nitrogen, and 15 g of carbon dioxide. calculate (a) the moles of each gas present, (b) the partial pressure exerted by each gas, (c) the total pressure exerted by the mixture, and (d) the percentage by volume of each gas in the mixture. you may assume ideal gas behavior

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:00
Which of the following did jj thompson discover about atoms? a)an atom has an internal structure. b) atoms are tiny indivisible particles. c)electrons orbit the nucleus of an atom. d) the nucleus of an atom contains protons and neutrons.
Answers: 2


Chemistry, 22.06.2019 19:00
Sum of brother and sisters age is 26. four times the brothers age is subtracted from three times the sisters age, the difference is 8. what are the ages of the brother and sister?
Answers: 1

Chemistry, 22.06.2019 21:00
One similarity and one difference between an element and a mixture of elements
Answers: 1
You know the right answer?
A30-liter volume of gas at 25°c contains 12 g of methane, 1 g of nitrogen, and 15 g of carbon dioxid...
Questions




English, 10.05.2021 16:30




Spanish, 10.05.2021 16:30


Mathematics, 10.05.2021 16:30





History, 10.05.2021 16:30

Mathematics, 10.05.2021 16:30



Physics, 10.05.2021 16:30