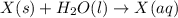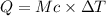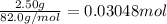Chemistry, 24.07.2019 16:10 christhegreat1
Acalorimeter contains 22.0 ml of water at 14.0 ∘c . when 2.50 g of x (a substance with a molar mass of 82.0 g/mol ) is added, it dissolves via the reaction x(s)+h2o(l)→x(aq) and the temperature of the solution increases to 28.0 ∘c . calculate the enthalpy change, δh, for this reaction per mole of x. assume that the specific heat of the resulting solution is equal to that of water [4.18 j/(g⋅∘c)], that density of water is 1.00 g/ml, and that no heat is lost to the calorimeter itself, nor to the surroundings.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Asolution of sodium hydroxide was titrated against a solution of sulfuric acid. how many moles of sodium hydroxide would react with 1 mole of sulfuric acid?
Answers: 2

Chemistry, 22.06.2019 09:00
Ineed to find the answer of this question because i dont understand it
Answers: 1

Chemistry, 22.06.2019 10:00
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1

Chemistry, 23.06.2019 00:30
What is bromine+calcium iodide--> calcium bromide +iodine balanced
Answers: 1
You know the right answer?
Acalorimeter contains 22.0 ml of water at 14.0 ∘c . when 2.50 g of x (a substance with a molar mass...
Questions





English, 19.03.2021 18:30

Social Studies, 19.03.2021 18:30

Chemistry, 19.03.2021 18:30

English, 19.03.2021 18:30

Biology, 19.03.2021 18:30



Mathematics, 19.03.2021 18:30

Mathematics, 19.03.2021 18:30






English, 19.03.2021 18:30