
You have two 466.0 ml aqueous solutions. solution a is a solution of silver nitrate, and solution b is a solution of potassium chromate. the masses of the solutes in each of the solutions are the same. when the solutions are added together, a blood-red precipitate forms. after the reaction has gone to completion, you dry the solid and find that it has a mass of 331.8 g. (a) calculate the concentration of the potassium ions in the original potassium chromate solution.(b) calculate the concentration of the chromate ions in the final solution

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which part of a feedback mechanism is able to monitor the conditions outside of cells and usually uses nerve cells to relay this information to an intergrating center
Answers: 2

Chemistry, 22.06.2019 10:00
In a water molecule, hydrogen and oxygen are held together by a(an) bond. a) double covalent b) ionic c) nonpolar covalent d) hydrogen e) polar covalent
Answers: 1

Chemistry, 22.06.2019 13:00
The molality of calcium chloride (cacl2) in an aqueous solution is 2.46 m. what is mole fraction of the solute?
Answers: 3

Chemistry, 23.06.2019 00:30
Gasoline has a density of 0.740 g/ml. if you have 328 grams of gasoline, what is the volume in milliliters?
Answers: 1
You know the right answer?
You have two 466.0 ml aqueous solutions. solution a is a solution of silver nitrate, and solution b...
Questions

SAT, 30.09.2019 07:30

Spanish, 30.09.2019 07:30

Mathematics, 30.09.2019 07:30


Mathematics, 30.09.2019 07:30

English, 30.09.2019 07:30




Mathematics, 30.09.2019 07:30

Mathematics, 30.09.2019 07:30


Business, 30.09.2019 07:30

Mathematics, 30.09.2019 07:30

Physics, 30.09.2019 07:30


Mathematics, 30.09.2019 07:30

Biology, 30.09.2019 07:30

Mathematics, 30.09.2019 07:30

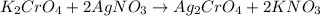
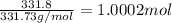
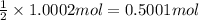 of silver nitrate.
of silver nitrate.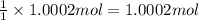 of potassium chromate
of potassium chromate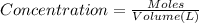
![[K^+]=\frac{2.0004 mol}{0.466 L}=4.2927 mol/L](/tpl/images/0127/0717/630d0.png)
![[CrO_4^{2+}]=\frac{1.0002 mol}{0.466 L+0.466L}=1.0731 mol/L](/tpl/images/0127/0717/e3c96.png)


