
Chemistry, 23.07.2019 06:20 rainbowboy9231
Agaseous compound is 78.14 percent boron and 21.86 percent hydrogen. at 27°c, 74.3 ml of the gas exerted a pressure of 1.12 atm. if the mass of the gas was 0.0934 g, what is its molecular formula?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Balance this equation: n2 + h2 > nh3, write the following molar ratios: n2 / n2 / nh3 h2 /
Answers: 1

Chemistry, 22.06.2019 06:30
The following reaction shows the products when sulfuric acid and aluminum hydroxide react. al(oh)3 + h2so4 → al2(so4)3 + h2o the table shows the calculated amounts of reactants and products when the reaction was conducted in a laboratory. sulfuric acid aluminum hydroxide initial amount of reactant 40 g 15 g theoretical yield of water from reactant 14.69 g 10.38 g what is the approximate amount of the leftover reactant? 11.73 g of sulfuric acid 10.33 g of sulfuric acid 11.12 g of aluminum hydroxide 13.67 g of aluminum hydroxide
Answers: 3

Chemistry, 22.06.2019 11:00
Which element would mostly likely have an electron affinity measuring closest to zero
Answers: 3

Chemistry, 23.06.2019 10:00
Abike ride event is 30 miles. a first aid tent is put at the 3/4 mark of the course. how many miles from the starting point is the first aid tent?
Answers: 1
You know the right answer?
Agaseous compound is 78.14 percent boron and 21.86 percent hydrogen. at 27°c, 74.3 ml of the gas exe...
Questions

Mathematics, 23.01.2022 03:50


Biology, 23.01.2022 03:50

Social Studies, 23.01.2022 03:50

History, 23.01.2022 03:50


Computers and Technology, 23.01.2022 03:50

Mathematics, 23.01.2022 03:50


Mathematics, 23.01.2022 03:50




History, 23.01.2022 03:50

Mathematics, 23.01.2022 03:50

Social Studies, 23.01.2022 03:50

Mathematics, 23.01.2022 03:50

Social Studies, 23.01.2022 04:00

Social Studies, 23.01.2022 04:00

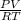
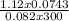
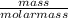
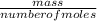
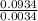
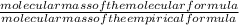
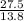 = 2
= 2

