(a) The pH of 0.1000 M propanoic acid (HC3H5O2) is 2.9.
(b) The pH of 0.1000 M sodium propanoate (NaC3H5O2) is 8.9.
(c) The pH of 0.1000 M propanoic acid (HC3H5O2) and 0.1000 M sodium propanoate (NaC3H5O2) is 4.9.
Further explanation:
(a)
Given information:
The value of acid ionization constant for propanoic acid is 1.3 x 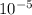 .
.
The initial concentration of propanoic acid is .
To calculate:
The pH of 0.1000 M propanoic acid solution.
Solution:
Propanoic acid is a weak acid. It ionizes partially in water as follows:
The expression for acid dissociation constant is,
…… (1)
Here,
is ionization constant of propanoic acid.
is the equilibrium concentration of propanoate ion.
is the equilibrium concentration of hydronium ion.
is the equilibrium concentration of propanoic acid.
ICE table (1):
Refer ICE table (1),
Substitute the values form the ICE table (1) in equation (1).
The approximation x is very small is valid. Therefore, the value of x can be neglected. Above equation can be modified as,
Rearrange above equation for x.
…… (2)
Substitute for in equation (2) to calculate the value of x.
Therefore, from the ICE table (1) the concentration of hydronium ion is,
.
The negative logarithm of hydronium ion concentration is defined as the pH of the solution. Mathematically,
…… (3)
Substitute for in equation (3) to calculate the pH of the solution.
(b)
Given information:
The value of acid ionization constant for propanoic acid is .
The initial concentration of sodium propanoate is .
To calculate:
The pH of 0.1000 M sodium propanoate solution.
Solution:
Sodium propanoate is conjugate base of weak propanoic acid. It undergoes hydrolysis in water to yield hydroxide ion in the solution as follows:
…… (4)
Propanoic acid is a weak acid. It ionizes partially in water as follows:
…… (5)
Dissociation reaction for water is written as follows:
…… (6)
From equation (4), (5), and (6) the relationship between and is,
…… (7)
Substitute for and for in equation (7).
ICE table (2):
The expression for base dissociation constant is,
…… (8)
Here,
is base ionization constant.
is the equilibrium concentration of propanoate ion.
is the equilibrium concentration of hydroxide ion.
is the equilibrium concentration of propanoic acid.
From the ICE table (2),
Substitute the values form the ICE table (2) in equation (8).
The approximation y is very small is valid. Therefore, the value of y can be neglected. Above equation can be modified as,
Rearrange above equation for y.
…… (9)
Substitute for in equation (9) to calculate the value of y.
Therefore, from the ICE table (2) the concentration of hydroxide ion is,
The negative logarithm of hydroxide ion concentration is defined as pOH of the solution. Mathematically,
…… (10)
Substitute for in equation (10) to calculate pOH of the solution.
The relation between pH and pOH is as follows:
…… (11)
Substitute 5.057 for pOH in equation (11) to calculate the pH of the solution.
(c)
Given information:
The value of acid ionization constant for propanoic acid is .
The initial concentration of sodium propanoate is .
The initial concentration of sodium propanoate is .
To calculate:
The pH of 0.1000 M sodium propanoate and 0.1000 M propanoic acid solution.
Solution:
Propanoic acid is a weak acid, and sodium propanoate is salt of the conjugate base of propanoic acid. Thus, propanoic acid and sodium propanoate will form a buffer system.
The pH of the buffer solution can be determined with the help of the Henderson-Hasselbalch equation. Mathematically,
For propanoic acid and sodium propanoate buffer system, the Henderson-Hasselbalch equation can be modified as,
…… (12)
The negative logarithm of acid ionization constant is equal to .
…… (13)
Substitute for in equation (13).
Substitute for , for and 4.9 for in equation (12).
Learn more:
1. About Henderson-Hasselbalch equation
2. Learn more about how to calculate moles of the base in given volume
Answer details:
Grade: Senior School
Subject: Chemistry
Chapter: Ionic equilibria
Keywords: ionic equilibrium, propanoic acid, sodium propanoate, ionization constant, weak acid, conjugate base, equilibrium concentration, hydronium ion, hydroxide ion, pH, pOH, ICE table, negative logarithm, buffer solution, Henderson-Hasselbalch equation, 0.1000 M, 4.9, 8.9, 2.9.




























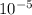 .
.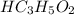 ⇄
⇄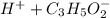
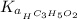 =
=![\frac{[H^+][C_3H_5O_2]^-}{[HC_3H_5O_2]}](/tpl/images/0122/2883/2da03.png) (4)
(4)
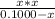 (5)
(5)
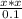
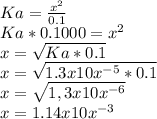
![pH= -Log [H^{+} ]\\pH=-Log(1.14x10^{-3} )\\pH=2.94](/tpl/images/0122/2883/928f2.png)
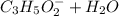 ⇄
⇄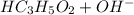 (6)
(6)
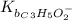 )
)
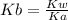 (7)
(7)
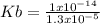
![\frac{[OH^-][HC_3H_5O_2]}{[C_3H_5O_2]}](/tpl/images/0122/2883/bf732.png) (11)
(11)
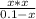 (12)
(12)
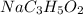 →
→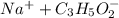 (14)
(14)
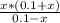 (19)
(19)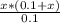
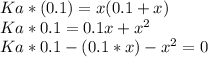
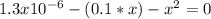 (20)
(20)
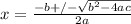 (21)
(21)
![pH= -Log [H^{+} ]\\pH=-Log(1.3x10^{-5} )\\pH=4.89](/tpl/images/0122/2883/1337f.png)


